The ubiquitin system
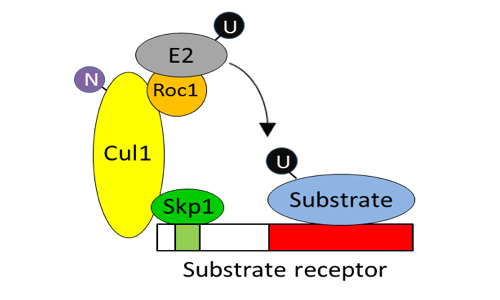
The ubiquitin system has emerged as a crucial regulator of many, if not all, biological processes. It consists of an unknown (~600) number of ubiquitin ligases that recognise specific substrates to trigger their subsequent ubiquitination. Given its importance and exquisite specificity, the ubiquitin system represents a very attractive target for viral manipulation. We are interested in understanding how viruses engage with such system and how they exploit, or inhibit, cellular ubiquitin ligases to their benefit. Such studies will provide key insights into the virus-host interplay and are likely to identify novel anti-viral therapeutic interventions.
Poxviruses are masters in the manipulation of the ubiquitin system, particularly the Cullin family of E3 ubiquitin ligases. Cullins are scaffold proteins that ubiquitylate specific substrates recognised by a series of substrate adaptors. Poxviruses are known to encode viral adaptors of Cullin-1, which origin is unclear, and Cullin-3 to redirect the Cullin ubiquitin ligase activity in a manner that is beneficial for the virus. Our work has recently demonstrated that poxviruses also encode adaptors of Cullin-2 and these adaptors are similar to cellular genes (Odon et al. 2018, J Virol, PMID 30258003). This suggests that poxviral Cullin-2 adaptors may represent the ancestral adaptor acquired from the host that eventually give rise to current poxviral Cullin-1 adaptors.
